Sterilized positioning instruments should be removed from the packages with the utmost care to maintain their sterility and prevent contamination. This article will delve into the proper techniques for removing sterilized positioning instruments from packages, ensuring their safe and effective use in various medical procedures.
The subsequent paragraphs will provide detailed information on sterilization protocols, package handling, and storage and maintenance of sterilized positioning instruments.
Sterilization Process and Standards
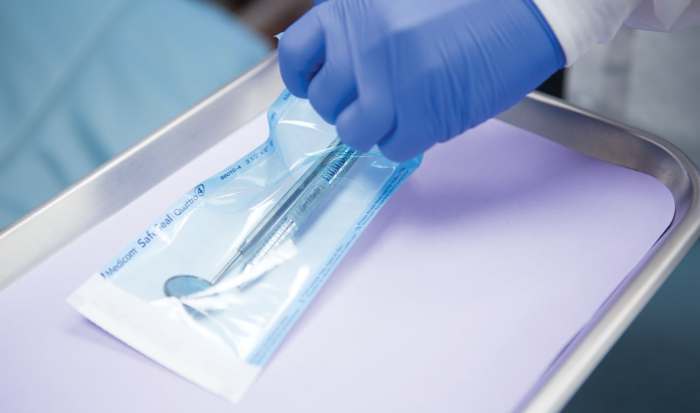
Sterilization is a crucial process in healthcare settings to eliminate microorganisms from medical devices and instruments, including positioning instruments. Adhering to standard protocols and guidelines for sterilization is essential to ensure patient safety and prevent infections.
Standard Protocols for Sterilizing Positioning Instruments
Standard protocols for sterilizing positioning instruments typically involve the following steps:
- Cleaning and disinfection to remove organic matter and microorganisms
- Rinsing and drying to eliminate residual cleaning agents and moisture
- Packaging in sterile containers to maintain sterility during storage and handling
- Using validated sterilization methods such as steam, ethylene oxide, or hydrogen peroxide gas
- Verifying sterility through biological indicators or other quality control measures
Importance of Adhering to Sterilization Guidelines, Sterilized positioning instruments should be removed from the packages
Adhering to sterilization guidelines is paramount for several reasons:
- Protects patients from infections and complications
- Maintains the integrity and functionality of instruments
- Complies with regulatory standards and accreditation requirements
- Prevents the spread of resistant microorganisms
Effective Sterilization Techniques
Effective sterilization techniques for positioning instruments include:
- Steam sterilization: Exposes instruments to high-pressure steam for a specific duration, effectively killing microorganisms
- Ethylene oxide sterilization: Uses a gas to penetrate and destroy microorganisms, suitable for instruments sensitive to heat or moisture
- Hydrogen peroxide gas sterilization: Employs a low-temperature gas to eliminate microorganisms, ideal for instruments with complex or delicate components
Package Integrity and Handling

Sterilized positioning instruments are typically packaged in sterile containers to maintain sterility during storage and handling. Proper handling procedures are crucial to prevent contamination and ensure the instruments remain sterile.
Types of Packaging for Sterilized Positioning Instruments
Common types of packaging used for sterilized positioning instruments include:
- Peel pouches: Single-use, flexible pouches that can be easily opened and closed
- Rigid containers: Durable containers made of plastic or metal, providing protection during storage and transportation
- Tyvek sleeves: Tear-resistant sleeves that cover instruments during sterilization and storage
Proper Handling Procedures for Sterilized Packages
Proper handling procedures for sterilized packages include:
- Inspecting packages for damage or tears before use
- Handling packages with clean hands or sterile gloves
- Storing packages in a clean, dry environment
- Avoiding prolonged exposure to moisture or direct sunlight
Consequences of Mishandling Sterilized Packages
Mishandling sterilized packages can compromise the sterility of instruments and increase the risk of infection. Consequences of mishandling include:
- Contamination of instruments with microorganisms
- Increased risk of surgical site infections
- Potential legal liability for healthcare providers
Removal and Usage

Safe removal and proper usage of sterilized positioning instruments are essential to maintain sterility and prevent contamination.
Safe Removal of Sterilized Positioning Instruments from Packages
To safely remove sterilized positioning instruments from packages:
- Wear sterile gloves and a clean gown
- Open packages in a sterile field or designated clean area
- Handle instruments only by their sterile surfaces, avoiding contact with non-sterile areas
- Transfer instruments directly to a sterile tray or field for immediate use
Importance of Using Sterile Gloves and Techniques
Using sterile gloves and techniques when handling sterilized instruments is crucial because:
- Prevents contamination of instruments with microorganisms from hands
- Maintains sterility of instruments during handling and use
- Reduces the risk of surgical site infections
Potential Risks Associated with Using Non-Sterile Instruments
Using non-sterile instruments poses significant risks, including:
- Increased risk of surgical site infections
- Potential for transmission of bloodborne pathogens
- Compromised patient safety and well-being
Storage and Maintenance: Sterilized Positioning Instruments Should Be Removed From The Packages
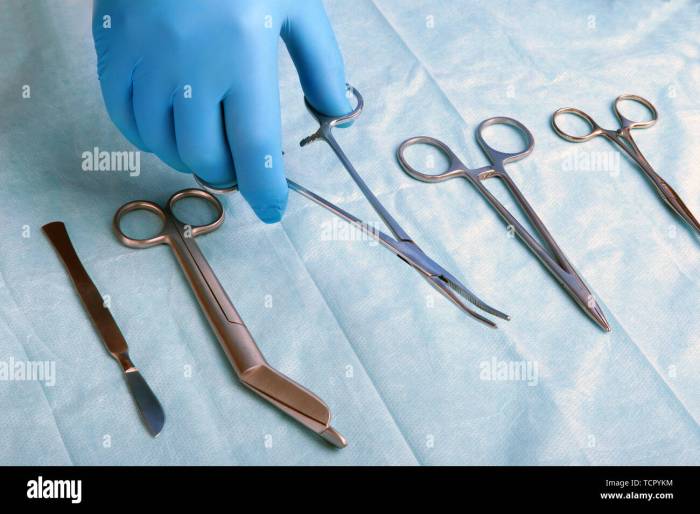
Proper storage and maintenance of sterilized positioning instruments are essential to ensure their longevity and continued sterility.
Proper Storage Conditions for Sterilized Positioning Instruments
Sterilized positioning instruments should be stored in a clean, dry, and temperature-controlled environment to maintain sterility and prevent damage:
- Store instruments in a designated storage area away from direct sunlight and moisture
- Maintain a temperature range of 15-25°C (59-77°F)
- Protect instruments from dust and other contaminants
Importance of Regular Maintenance and Inspection of Instruments
Regular maintenance and inspection of sterilized instruments are crucial for several reasons:
- Ensures proper functioning and accuracy of instruments
- Identifies potential damage or wear that could compromise sterility
- Extends the lifespan of instruments
Guidelines for Tracking and Monitoring Sterilized Instruments
Tracking and monitoring sterilized instruments is essential to ensure their proper use and maintenance:
- Establish a system to track instrument usage and sterilization cycles
- Maintain records of sterilization dates and expiration dates
- Regularly inspect instruments for damage or defects
Frequently Asked Questions
Why is it important to remove sterilized positioning instruments from packages properly?
Removing sterilized positioning instruments from packages properly helps maintain their sterility and prevents contamination, ensuring patient safety during medical procedures.
What are the consequences of mishandling sterilized packages?
Mishandling sterilized packages can compromise the sterility of the instruments, potentially leading to infections and other complications in patients.
How should sterilized positioning instruments be stored?
Sterilized positioning instruments should be stored in a clean, dry, and temperature-controlled environment to maintain their sterility and prevent damage.